Patients Deserve Safe Servicing
The medical imaging equipment we rely on to diagnose and monitor cancer and other life-threatening illnesses must be periodically serviced and maintained to ensure that it is working properly. Without proper regulation, medical device servicing businesses could put technicians and patients at risk for serious injury or result in poor image quality, leading to a delayed or missed diagnosis, multiple imaging procedures, and increased healthcare costs.
To ensure safety, the individuals servicing these devices should be highly trained and their employers appropriately regulated – but unfortunately, that is not always the case.
Servicing technicians who work for original equipment manufacturers (OEMs) receive detailed training, shadow more experienced technicians, and return for continuing education on new or updated devices. Further, these OEMs are regulated by the FDA while their third-party servicer business competitors operate wholly outside of any oversight. Third-party servicers are not required to undergo the same rigorous training programs as OEMs, do not have to register with the FDA, and are not required to implement quality or safety controls.
The result of this lack of oversight can be shocking, as you can see in the examples below:

This CT injector was being maintained by a third-party business that allowed foreign material to build upon the control panel and perhaps elsewhere in the device, creating potential risks for corrosion or contamination.

The third-party servicing business did not replace these CT filters for several years. In fact, after an investigation, it turned out that the third-party did not even know these filters existed! Clogged filters create a risk of the gantry overheating, potentially resulting in premature failure of electronics and high voltage controls. Image quality can also be affected, resulting in artifacts, as the detector modules are very heat sensitive.
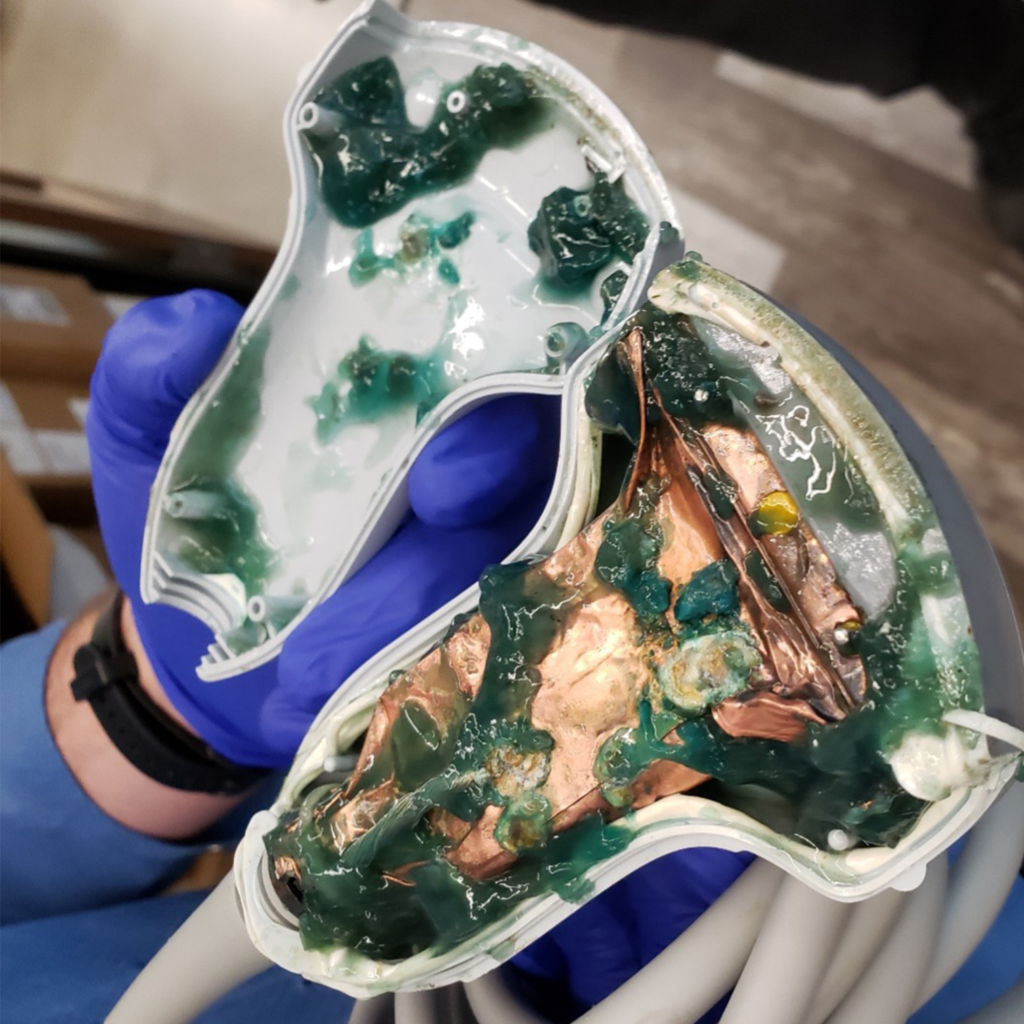
A third-party servicing business failed to properly seal this transducer after a repair, allowing ultrasound gel to seep into the unit, creating risks for contamination, poor image quality, and electrical shock for the sonographer.
Fortunately, there is a solution: The FDA must increase accountability and transparency of the third-party service industry to ensure the safe and effective operation of all medical devices.
Visit the Patients Deserve Safe Servicing website to learn more about the risks and solutions to this serious issue.
More Articles
Mark’s PET Imaging Story
In March 2011, I was diagnosed with prostate cancer with a Gleason score of 7 (out of 10).…
Read MoreEnhancing Patient Safety: An Insight into Medical Device Servicing Standards
Wes Bonebright has over two decades of experience in the medical device servicing industry. As an engineer, Bonebright…
Read MorePatient Spotlight: Bobby Krause
Just a few years ago, Bobby was a 39-year-old marketing executive who reveled in the joys of parenthood,…
Read MoreWhat You Need to Know About Breast Cancer Screening Guidelines
It’s alarming that breast cancer rates are on the rising among younger women. While current guidance recommends mammograms…
Read More