Real Risks of Unregulated Third-Party Servicing
Medical imaging has revolutionized healthcare from the start but especially over the past 30 years. Today, patients and healthcare providers depend on the safe and effective operation of medical imaging devices to diagnose, monitor and guide treatment of cancer and other diseases.
To ensure that patients are receiving safe and accurate scans, the individuals servicing these devices must be highly trained and following “best practices” processes. Accordingly, their employers must be regulated.
Servicing technicians who work for original equipment manufacturers receive extensive training, shadow more experienced technicians, and return for continuing education on new or updated devices – all under the oversight of the Food and Drug Administration (FDA). However, many medical devices are maintained by unregulated third-party servicers who are not required to undergo the same rigorous training program and do not have to register with FDA. This means that some of these highly complicated machines are serviced by businesses that are not required to meet any proper quality and safety Standards. This means their work is done with no oversight.
Without proper oversight, there is an increased risk for serious patient and operator safety and device performance issues. Below are some examples of what happens when medical imaging equipment is improperly serviced by unregulated third parties:
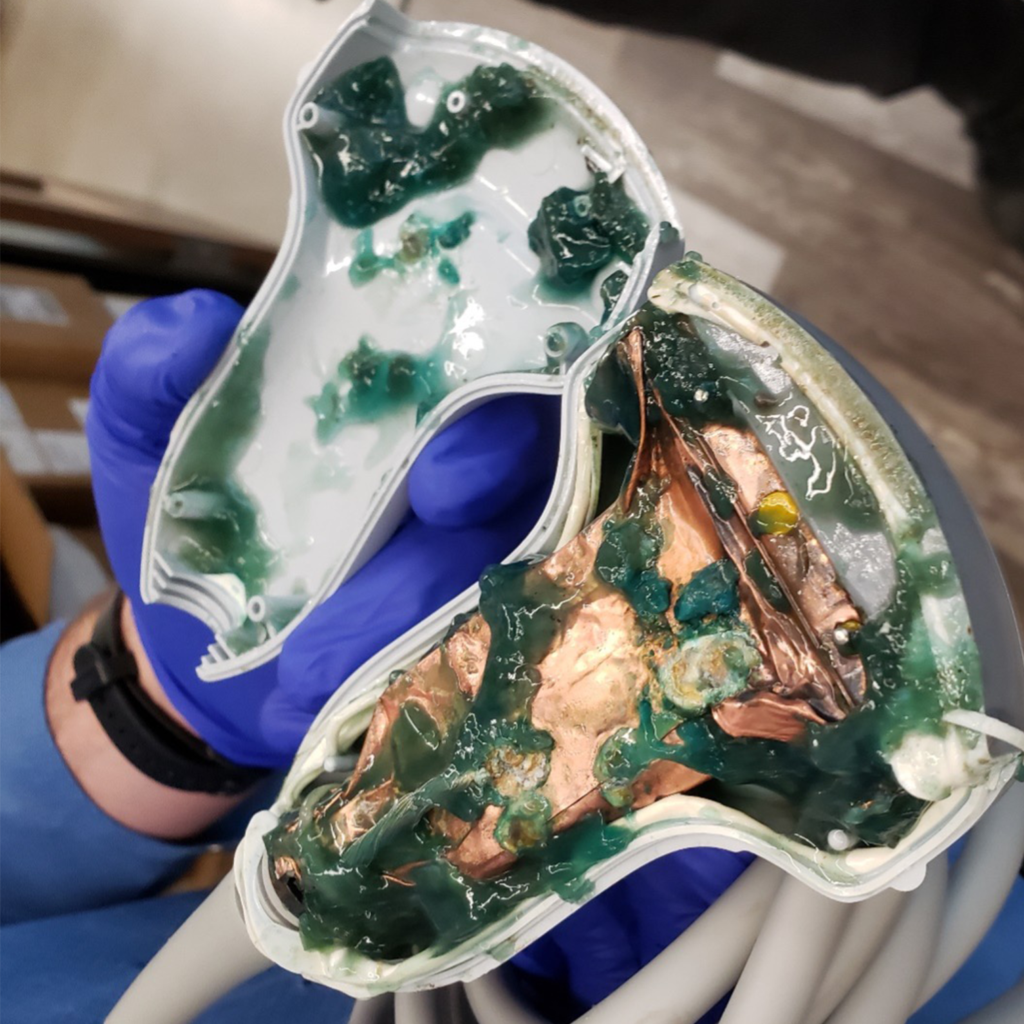
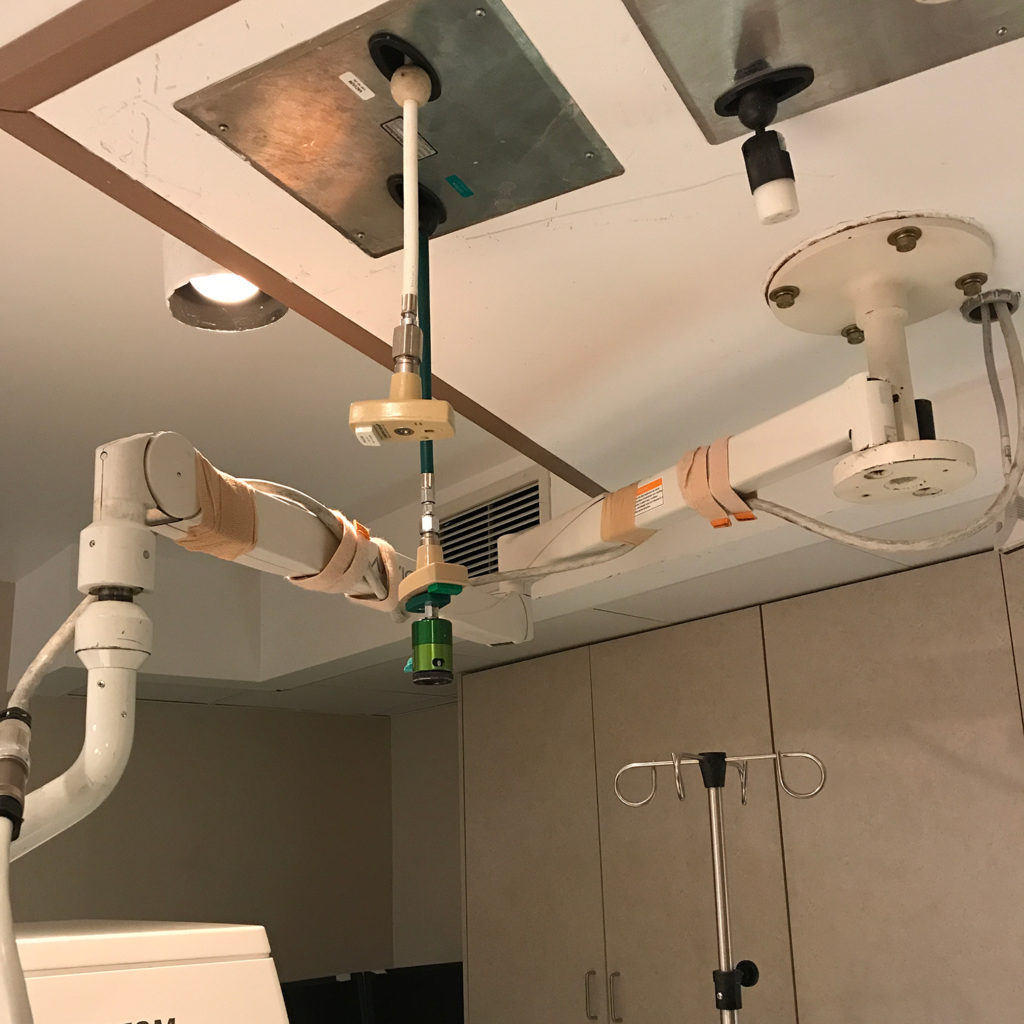


Proper oversight by FDA can help prevent these dangerous situations. FDA should hold all servicing businesses to the same quality, safety, and regulatory requirements, including proper training, implementation of quality and safety controls, adverse event reporting, and registration, to ensure that patients are receiving safe and accurate scans.
More Articles
National Oral Cancer Month: Early Detection Saves Lives
April is Oral Cancer Awareness Month – an opportunity to raise awareness about the serious and deadly effects…
Read MoreHealth Tech Investment Act Can Improve Patient Care
The future of healthcare is here – and Artificial Intelligence (AI) is at the forefront. Already, AI-enabled medical…
Read MoreNational Colorectal Cancer Awareness Month: The Importance of Early Detection
Every March, we recognize National Colorectal Cancer Awareness Month as an opportunity to raise awareness about this all-too-common…
Read MoreLung Cancer Awareness Month: Why AI-Enabled Imaging Tools Matter
This year, an estimated 234,580 Americans will be diagnosed with lung cancer. This Lung Cancer Awareness Month, we…
Read More